A Stable Coordination Complex of Rh(IV) in an N,O-Donor Environment
http://pubs.acs.org/doi/abs/10.1021/jacs.5b12148
Shashi B. Sinha, Dimitar Y. Shopov, Liam S. Sharninghausen, David J. Vinyard, Brandon Q. Mercado, Gary W. Brudvig*, and Robert H. Crabtree*
Department of Chemistry, Yale University, 225 Prospect Street, New Haven, Connecticut 06520, United States
J. Am. Chem. Soc., Article ASAP
DOI: 10.1021/jacs.5b12148
Publication Date (Web): December 7, 2015
Copyright © 2015 American Chemical Society
Abstract: We describe facial and meridional isomers of [RhIII(pyalk)3], as well as meridional [RhIV(pyalk)3]+ {pyalk =2-(2-pyridyl)-2-propanoate}, the first coordination complex in an N,O-donor environment to show a clean, reversible RhIII/IV redox couple and to have a stable Rh(IV) form, which we characterize by EPR and UV–visible spectroscopy as well as X-ray crystallography. The unprecedented stability of the Rh(IV) species is ascribed to the exceptional donor strength of the ligands, their oxidation resistance, and the meridional coordination geometry.
Tuesday, December 15, 2015
Friday, December 11, 2015
Polyannulated Bis(N-heterocyclic carbene)palladium Pincer Complexes for Electrocatalytic CO2 Reduction
Polyannulated Bis(N-heterocyclic carbene)palladium Pincer
Complexes for Electrocatalytic CO2 Reduction
Jeffrey A. Therrien, Michael O. Wolf,* and Brian O. Patrick
Department of Chemistry, University of British Columbia, Vancouver, British Columbia V6T 1Z1, Canada
Inorg. Chem. DOI: 10.1021/acs.inorgchem.5b01698
Abstract:
Phenanthro- and pyreno-annulated N-heterocyclic carbenes (NHCs) have been incorporated into lutidine-linked bisNHC Pd pincer complexes to investigate the effect of these polyannulated NHCs on the ability of the complexes to electrochemically reduce CO2 to CO in the presence of 2,2,2-trifluoroacetic acid and 2,2,2-trifluoroethanol as proton sources. These complexes are screened for their ability to reduce CO2 and modeled using density functional theory calculations, where the annulated phenanthrene and pyrene moieties are shown to be additional sites for redox activity in the pincer ligand, enabling increased electron donation. Electrochemical and computational studies are used to gain an understanding of the chemical significance of redox events for complexes of this type, highlighting the importance of anion binding and dissociation.
TOC:
Jeffrey A. Therrien, Michael O. Wolf,* and Brian O. Patrick
Department of Chemistry, University of British Columbia, Vancouver, British Columbia V6T 1Z1, Canada
Inorg. Chem. DOI: 10.1021/acs.inorgchem.5b01698
Abstract:
Phenanthro- and pyreno-annulated N-heterocyclic carbenes (NHCs) have been incorporated into lutidine-linked bisNHC Pd pincer complexes to investigate the effect of these polyannulated NHCs on the ability of the complexes to electrochemically reduce CO2 to CO in the presence of 2,2,2-trifluoroacetic acid and 2,2,2-trifluoroethanol as proton sources. These complexes are screened for their ability to reduce CO2 and modeled using density functional theory calculations, where the annulated phenanthrene and pyrene moieties are shown to be additional sites for redox activity in the pincer ligand, enabling increased electron donation. Electrochemical and computational studies are used to gain an understanding of the chemical significance of redox events for complexes of this type, highlighting the importance of anion binding and dissociation.
TOC:
Standard Reduction Potentials for Oxygen and Carbon Dioxide Couples in Acetonitrile and N,N‑Dimethylformamide
Standard Reduction Potentials for Oxygen and Carbon Dioxide
Couples in Acetonitrile and N,N‑Dimethylformamide
Michael L. Pegis,† John A. S. Roberts,‡,∥ Derek J. Wasylenko,§,⊥ Elizabeth A. Mader,† Aaron M. Appel,*,‡ and James M. Mayer*,†
† Department of Chemistry, Yale University, PO Box 208107, New Haven, Connecticut 06520-8107, United States ‡ Center for Molecular Electrocatalysis, Pacific Northwest National Laboratory, P.O. Box 999 (K2-57), Richland, Washington 99352, United States § Department of Chemistry, University of Washington, Campus Box 351700, Seattle, Washington 98195-1700, United States
Inorg. Chem. DOI: 10.1021/acs.inorgchem.5b02136
Abstract:
A variety of next-generation energy processes utilize the electrochemical interconversions of dioxygen and water as the oxygen reduction reaction (ORR) and the oxygen evolution reaction (OER). Reported here are the first estimates of the standard reduction potential of the O2 + 4e − + 4H+ ⇋ 2H2O couple in organic solvents. The values are +1.21 V in acetonitrile (MeCN) and +0.60 V in N,N-dimethylformamide (DMF), each versus the ferrocenium/ferrocene couple (Fc+/0) in the respective solvent (as are all of the potentials reported here). The potentials have been determined using a thermochemical cycle that combines the free energy for transferring water from aqueous solution to organic solvent, −0.43 kcal mol−1 for MeCN and −1.47 kcal mol−1 for DMF, and the potential of the H+ /H2 couple, − 0.028 V in MeCN and −0.662 V in DMF. The H+ /H2 couple in DMF has been directly measured electrochemically using the previously reported procedure for the MeCN value. The thermochemical approach used for the O2/H2O couple has been extended to the CO2/CO and CO2/CH4 couples to give values of −0.12 and +0.15 V in MeCN and −0.73 and −0.48 V in DMF, respectively. Extensions to other reduction potentials are discussed. Additionally, the free energy for transfer of protons from water to organic solvent is estimated as +14 kcal mol−1 for acetonitrile and +0.6 kcal mol−1 for DMF.
TOC:
Michael L. Pegis,† John A. S. Roberts,‡,∥ Derek J. Wasylenko,§,⊥ Elizabeth A. Mader,† Aaron M. Appel,*,‡ and James M. Mayer*,†
† Department of Chemistry, Yale University, PO Box 208107, New Haven, Connecticut 06520-8107, United States ‡ Center for Molecular Electrocatalysis, Pacific Northwest National Laboratory, P.O. Box 999 (K2-57), Richland, Washington 99352, United States § Department of Chemistry, University of Washington, Campus Box 351700, Seattle, Washington 98195-1700, United States
Inorg. Chem. DOI: 10.1021/acs.inorgchem.5b02136
Abstract:
A variety of next-generation energy processes utilize the electrochemical interconversions of dioxygen and water as the oxygen reduction reaction (ORR) and the oxygen evolution reaction (OER). Reported here are the first estimates of the standard reduction potential of the O2 + 4e − + 4H+ ⇋ 2H2O couple in organic solvents. The values are +1.21 V in acetonitrile (MeCN) and +0.60 V in N,N-dimethylformamide (DMF), each versus the ferrocenium/ferrocene couple (Fc+/0) in the respective solvent (as are all of the potentials reported here). The potentials have been determined using a thermochemical cycle that combines the free energy for transferring water from aqueous solution to organic solvent, −0.43 kcal mol−1 for MeCN and −1.47 kcal mol−1 for DMF, and the potential of the H+ /H2 couple, − 0.028 V in MeCN and −0.662 V in DMF. The H+ /H2 couple in DMF has been directly measured electrochemically using the previously reported procedure for the MeCN value. The thermochemical approach used for the O2/H2O couple has been extended to the CO2/CO and CO2/CH4 couples to give values of −0.12 and +0.15 V in MeCN and −0.73 and −0.48 V in DMF, respectively. Extensions to other reduction potentials are discussed. Additionally, the free energy for transfer of protons from water to organic solvent is estimated as +14 kcal mol−1 for acetonitrile and +0.6 kcal mol−1 for DMF.
TOC:
Anhydrous Tetramethylammonium Fluoride for Room-Temperature SNAr Fluorination
Anhydrous Tetramethylammonium Fluoride for Room-Temperature
SNAr Fluorination
Sydonie D. Schimler,† Sarah J. Ryan,† Douglas C. Bland,‡ John E. Anderson,‡ and Melanie S. Sanford*,†
† Department of Chemistry, University of Michigan, 930 North University Avenue, Ann Arbor, Michigan 48109, United States ‡ Process Science, Dow Chemical Company, 1710 Building, Midland, Michigan 48674, United States
J. Org. Chem. DOI: 10.1021/acs.joc.5b02075
http://pubs.acs.org/doi/pdf/10.1021/acs.joc.5b02075
Abstract:
This paper describes the room-temperature SNAr fluorination of aryl halides and nitroarenes using anhydrous tetramethylammonium fluoride (NMe4F). This reagent effectively converts aryl-X (X = Cl, Br, I, NO2, OTf) to aryl-F under mild conditions (often room temperature). Substrates for this reaction include electron-deficient heteroaromatics (22 examples) and arenes (5 examples). The relative rates of the reactions vary with X as well as with the structure of the substrate. However, in general, substrates bearing X = NO2 or Br react fastest. In all cases examined, the yields of these reactions are comparable to or better than those obtained with CsF at elevated temperatures (i.e., more traditional halex fluorination conditions). The reactions also afford comparable yields on scales ranging from 100 mg to 10 g. A cost analysis is presented, which shows that fluorination with NMe4F is generally more costeffective than fluorination with CsF.
TOC:
Sydonie D. Schimler,† Sarah J. Ryan,† Douglas C. Bland,‡ John E. Anderson,‡ and Melanie S. Sanford*,†
† Department of Chemistry, University of Michigan, 930 North University Avenue, Ann Arbor, Michigan 48109, United States ‡ Process Science, Dow Chemical Company, 1710 Building, Midland, Michigan 48674, United States
J. Org. Chem. DOI: 10.1021/acs.joc.5b02075
http://pubs.acs.org/doi/pdf/10.1021/acs.joc.5b02075
Abstract:
This paper describes the room-temperature SNAr fluorination of aryl halides and nitroarenes using anhydrous tetramethylammonium fluoride (NMe4F). This reagent effectively converts aryl-X (X = Cl, Br, I, NO2, OTf) to aryl-F under mild conditions (often room temperature). Substrates for this reaction include electron-deficient heteroaromatics (22 examples) and arenes (5 examples). The relative rates of the reactions vary with X as well as with the structure of the substrate. However, in general, substrates bearing X = NO2 or Br react fastest. In all cases examined, the yields of these reactions are comparable to or better than those obtained with CsF at elevated temperatures (i.e., more traditional halex fluorination conditions). The reactions also afford comparable yields on scales ranging from 100 mg to 10 g. A cost analysis is presented, which shows that fluorination with NMe4F is generally more costeffective than fluorination with CsF.
TOC:
Wednesday, December 9, 2015
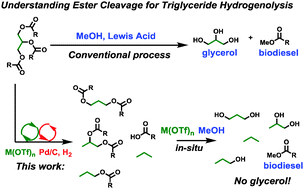
Mono- and tri-ester hydrogenolysis using tandem catalysis. Scope and mechanism
Mono- and tri-ester hydrogenolysis using tandem catalysis. Scope and mechanism
Tracy L. Lohr,a Zhi Li,a Rajeev S. Assary,b Larry A. Curtissbc and Tobin J. Marks*a
Energy Environ. Sci., 2016, Advance Article
DOI: 10.1039/C5EE03256C
Received 23 Oct 2015, Accepted 26 Nov 2015
First published online 26 Nov 2015
Abstract: The scope and mechanism of thermodynamically leveraged ester RC(O)O–R′ bond hydrogenolysis by tandem metal triflate + supported Pd catalysts are investigated both experimentally and theoretically by DFT and energy span analysis. This catalytic system has a broad scope, with relative cleavage rates scaling as, tertiary > secondary > primary ester at 1 bar H2, yielding alkanes and carboxylic acids with high conversion and selectivity. Benzylic and allylic esters display the highest activity. The rate law is ν = k[M(OTf)n]1[ester]0[H2]0 with an H/D kinetic isotope effect = 6.5 ± 0.5, implying turnover-limiting C–H scission following C–O cleavage, in agreement with theory. Intermediate alkene products are then rapidly hydrogenated. Applying this approach with the very active Hf(OTf)4 catalyst to bio-derived triglycerides affords near-quantitative yields of C3 hydrocarbons rather than glycerol. From model substrates, it is found that RC(O)O–R′ cleavage rates are very sensitive to steric congestion and metal triflate identity. For triglycerides, primary/external glyceryl CH2–O cleavage predominates over secondary/internal CH–O cleavage, with the latter favored by less acidic or smaller ionic radius metal triflates, raising the diester selectivity to as high as 48% with Ce(OTf)3.
Tuesday, December 8, 2015
Fighting Fenton Chemistry: A Highly Active Iron(III) Tetracarbene Complex in Epoxidation Catalysis
Jens W. Kück, Markus R. Anneser, Benjamin Hofmann, Dr. Alexander Pöthig, Dr. Mirza Cokoja, Prof. Dr. Fritz E. Kühn
http://onlinelibrary.wiley.com/doi/10.1002/cssc.201500930/full
Abstract
Organometallic Fe complexes with exceptionally high activities in homogeneous epoxidation catalysis are reported. The compounds display FeII and FeIII oxidation states and bear a tetracarbene ligand. The more active catalyst exhibits activities up to 183 000 turnovers per hour at room temperature and turnover numbers of up to 4300 at −30 °C. For the FeIII complex, a decreased Fenton-type reactivity is observed compared with FeII catalysts reported previously as indicated by a substantially lower H2O2 decomposition and higher (initial) turnover frequencies. The dependence of the catalyst performance on the catalyst loading, substrate, water addition, and the oxidant is investigated. Under all applied conditions, the advantageous nature of the use of the FeIII complex is evident.http://onlinelibrary.wiley.com/doi/10.1002/cssc.201500930/full
A manganese catalyst for highly reactive yet chemoselective intramolecular C(sp3)–H amination
Shauna M. Paradine, Jennifer R. Griffin, Jinpeng Zhao, Aaron L. Petronico, Shannon M. Miller, & M. Christina White
Nature Chemistry 7, 987–994 doi:10.1038/nchem.2366 Received Accepted Published online
A steric tethering approach enables palladium-catalysed C–H activation of primary amino alcohols
Jonas Calleja, Daniel Pla, Timothy W. Gorman, Victoriano Domingo, Benjamin Haffemayer, & Matthew J. Gaunt
Nature Chemistry 7, 1009–1016 doi:10.1038/nchem.2367 Received Accepted Published online
Abstract
Aliphatic primary amines are a class of chemical feedstock essential to the synthesis of higher-order nitrogen-containing molecules, commonly found in biologically active compounds and pharmaceutical agents. New methods for the construction of complex amines remain a continuous challenge to synthetic chemists. Here, we outline a general palladium-catalysed strategy for the functionalization of aliphatic C–H bonds within amino alcohols, an important class of small molecule. Central to this strategy is the temporary conversion of catalytically incompatible primary amino alcohols into hindered secondary amines that are capable of undergoing a sterically promoted palladium-catalysed C–H activation. Furthermore, a hydrogen bond between amine and catalyst intensifies interactions around the palladium and orients the aliphatic amine substituents in an ideal geometry for C–H activation. This catalytic method directly transforms simple, easily accessible amines into highly substituted, functionally concentrated and structurally diverse products, and can streamline the synthesis of biologically important amine-containing molecules.http://www.nature.com/nchem/journal/v7/n12/full/nchem.2367.html
Wednesday, December 2, 2015
Dual Role of Pyrrolidine and Cooperative Pyrrolidine/Pyrrolidinium Effect in Nitrone Formation
http://pubs.acs.org/doi/abs/10.1021/acscatal.5b01726
Sara Morales, Fernando G. Guijarro, Inés Alonso, José Luis García Ruano*, and M. Belén Cid*
Department of Organic Chemistry, Universidad Autónoma de Madrid, Cantoblanco, 28049 Madrid, Spain
ACS Catal., 2016, 6, pp 84–91
DOI: 10.1021/acscatal.5b01726
Publication Date (Web): November 12, 2015
Sara Morales, Fernando G. Guijarro, Inés Alonso, José Luis García Ruano*, and M. Belén Cid*
Department of Organic Chemistry, Universidad Autónoma de Madrid, Cantoblanco, 28049 Madrid, Spain
ACS Catal., 2016, 6, pp 84–91
DOI: 10.1021/acscatal.5b01726
Publication Date (Web): November 12, 2015
Abstract:
The formation of nitrones by direct condensation between equimolecular amounts of N-substituted hydroxylamine hydrochlorides and aromatic or aliphatic aldehydes is efficiently promoted by pyrrolidine in a matter of minutes under very mild conditions in almost quantitative yields after a simple filtration through a short pad of silica gel. According to theoretical, spectroscopic, and experimental studies, this success is due to the ability of pyrrolidine to liberate the hydrochloride of the hydroxylamine and catalyze the reaction via iminium activation ion. Moreover, a cooperative pyrrolidine/pyrrolidinium chloride effect facilitates several steps of the catalytic cycle through proton transfer without hampering the nucleophilicity of the hydroxylamine by protonation.
Keywords: nitrone preparation; iminiumactivation; cooperative; DFT calculations; pyrrolidine/pyrrolidinium
The formation of nitrones by direct condensation between equimolecular amounts of N-substituted hydroxylamine hydrochlorides and aromatic or aliphatic aldehydes is efficiently promoted by pyrrolidine in a matter of minutes under very mild conditions in almost quantitative yields after a simple filtration through a short pad of silica gel. According to theoretical, spectroscopic, and experimental studies, this success is due to the ability of pyrrolidine to liberate the hydrochloride of the hydroxylamine and catalyze the reaction via iminium activation ion. Moreover, a cooperative pyrrolidine/pyrrolidinium chloride effect facilitates several steps of the catalytic cycle through proton transfer without hampering the nucleophilicity of the hydroxylamine by protonation.
Keywords: nitrone preparation; iminiumactivation; cooperative; DFT calculations; pyrrolidine/pyrrolidinium
Synthesis, characterisation, and catalytic evaluation of hierarchical faujasite zeolites: milestones, challenges, and future directions†
Synthesis, characterisation, and catalytic
evaluation of hierarchical faujasite zeolites:
milestones, challenges, and future directions†
D. Verboekend,*a N. Nuttens,a R. Locus,a J. Van Aelst,a P. Verolme,b J. C. Groen,b J. Pe´rez-Ramı´rezc and B. F. Selsa
Chem. Soc. Rev. http://pubs.rsc.org/en/content/articlepdf/2016/cs/c5cs00520e?page=search
Abstract:
Faujasite (X, Y, and USY) zeolites represent one of the most widely-applied and abundant catalysts and sorbents in the chemical industry. In the last 5 years substantial progress was made in the synthesis, characterisation, and catalytic exploitation of hierarchically-structured variants of these zeolites. Hererin, we provide an overview of these contributions, highlighting the main advancements regarding the evaluation of the nature and functionality of introduced secondary porosity. The novelty, efficiency, versatility, and sustainability of the reported bottom-up and (predominately) top-down strategies are discussed. The crucial role of the relative stability of faujasites in aqueous media is highlighted. The interplay between the physico-chemical properties of the hierarchical zeolites and their use in petrochemical and biomass-related catalytic processes is assessed.
D. Verboekend,*a N. Nuttens,a R. Locus,a J. Van Aelst,a P. Verolme,b J. C. Groen,b J. Pe´rez-Ramı´rezc and B. F. Selsa
Chem. Soc. Rev. http://pubs.rsc.org/en/content/articlepdf/2016/cs/c5cs00520e?page=search
Abstract:
Faujasite (X, Y, and USY) zeolites represent one of the most widely-applied and abundant catalysts and sorbents in the chemical industry. In the last 5 years substantial progress was made in the synthesis, characterisation, and catalytic exploitation of hierarchically-structured variants of these zeolites. Hererin, we provide an overview of these contributions, highlighting the main advancements regarding the evaluation of the nature and functionality of introduced secondary porosity. The novelty, efficiency, versatility, and sustainability of the reported bottom-up and (predominately) top-down strategies are discussed. The crucial role of the relative stability of faujasites in aqueous media is highlighted. The interplay between the physico-chemical properties of the hierarchical zeolites and their use in petrochemical and biomass-related catalytic processes is assessed.
Heterogeneous chemistry and reaction dynamics of the atmospheric oxidants, O3, NO3, and OH, on organic surfaces
Heterogeneous chemistry and reaction dynamics
of the atmospheric oxidants, O3, NO3, and OH,
on organic surfaces
Robert C. Chapleski Jr., Yafen Zhang, Diego Troya and John R. Morris*
Chem. Soc. Rev. http://pubs.rsc.org/en/content/articlepdf/2016/cs/c5cs00375j?page=search
Abstract:
Heterogeneous chemistry of the most important atmospheric oxidants, O3, NO3, and OH, plays a central role in regulating atmospheric gas concentrations, processing aerosols, and aging materials. Recent experimental and computational studies have begun to reveal the detailed reaction mechanisms and kinetics for gas-phase O3, NO3, and OH when they impinge on organic surfaces. Through new research approaches that merge the fields of traditional surface science with atmospheric chemistry, researchers are developing an understanding for how surface structure and functionality affect interfacial chemistry with this class of highly oxidizing pollutants. Together with future research initiatives, these studies will provide a more complete description of atmospheric chemistry and help others more accurately predict the properties of aerosols, the environmental impact of interfacial oxidation, and the concentrations of tropospheric gases.
Robert C. Chapleski Jr., Yafen Zhang, Diego Troya and John R. Morris*
Chem. Soc. Rev. http://pubs.rsc.org/en/content/articlepdf/2016/cs/c5cs00375j?page=search
Abstract:
Heterogeneous chemistry of the most important atmospheric oxidants, O3, NO3, and OH, plays a central role in regulating atmospheric gas concentrations, processing aerosols, and aging materials. Recent experimental and computational studies have begun to reveal the detailed reaction mechanisms and kinetics for gas-phase O3, NO3, and OH when they impinge on organic surfaces. Through new research approaches that merge the fields of traditional surface science with atmospheric chemistry, researchers are developing an understanding for how surface structure and functionality affect interfacial chemistry with this class of highly oxidizing pollutants. Together with future research initiatives, these studies will provide a more complete description of atmospheric chemistry and help others more accurately predict the properties of aerosols, the environmental impact of interfacial oxidation, and the concentrations of tropospheric gases.
Sunday, November 22, 2015
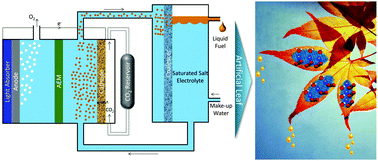
Design of an artificial photosynthetic system for production of alcohols in high concentration from CO2
Design of an artificial photosynthetic system for production of alcohols in high concentration from CO2
Meenesh R. Singha, Alexis T. Bell
Energy Environ. Sci., 2015, Advance Article
DOI: 10.1039/C5EE02783G
Received 10 Sep 2015, Accepted 06 Nov 2015
First published online 06 Nov 2015
Abstract: Artificial photosynthesis of liquid fuels is a potential source for clean energy. Alcohols are particularly attractive products because of their high energy density and market value per amount of energy input. The major challenges in photo/electrochemical synthesis of alcohols from sunlight, water and CO2 are low product selectivity, high membrane fuel-crossover losses, and high cost of product separation from the electrolyte. Here we propose an artificial photosynthesis scheme for direct synthesis and separation to almost pure ethanol with minimum product crossover using saturated salt electrolytes. The ethanol produced in the saturated salt electrolytes can be readily phase separated into a microemulsion, which can be collected as pure products in a liquid–liquid extractor. A novel design of an integrated artificial photosynthetic system is proposed that continuously produces >90 wt% pure ethanol using a polycrystalline copper cathode at a current density of 0.85 mA cm−2. The annual production rate of >90 wt% ethanol using such a photosynthesis system operating at 10 mA cm−2 (12% solar-to-fuel (STF) efficiency) can be 15.27 million gallons per year per square kilometer, which corresponds to 7% of the industrial ethanol production capacity of California.
Recent developments in the preparation of N-heterocycles using Pd-catalyzed C-H activation
Recent developments in the preparation of N-heterocycles using Pd-catalyzed C-H activation
http://www.sciencedirect.com/science/article/pii/S0022328X15301935
Tanveer Mahamad Ali Shaikha, Fung-E. Honga
doi:10.1016/j.jorganchem.2015.10.022
Journal of Organometallic Chemistry, In Press, Accepted Manuscript
Abstract: The direct and selective activation of C-H bonds for the synthesis of N-heterocycles (C-N bonds) is an important areain modern synthetic chemistry due to its wide application in both in academics and industry. These compounds are frequently found as backbones in bioactive molecules and fine chemicals and are often used as organocatalysts or as auxiliary ligands in transition metal complexes assisted catalyses. Various kinds of palladium-containing complexes as active catalysts in the C(sp3)-H or C(sp2)-H bond activation have attracted increasing attention because of their accessibility and catalytic efficiencies. This review summarizes the recent developments in this area which employs Pd0-PdIV as versatile catalyst precursors to the syntheses of N-heterocycles. The N-heterocycles, including five- and six-membered rings, are described separately. Furthermore, some of the asymmetric C-H bond activation and mechanistic aspects of these catalytic reactions are discussed.
http://www.sciencedirect.com/science/article/pii/S0022328X15301935
Tanveer Mahamad Ali Shaikha, Fung-E. Honga
doi:10.1016/j.jorganchem.2015.10.022
Journal of Organometallic Chemistry, In Press, Accepted Manuscript
Abstract: The direct and selective activation of C-H bonds for the synthesis of N-heterocycles (C-N bonds) is an important areain modern synthetic chemistry due to its wide application in both in academics and industry. These compounds are frequently found as backbones in bioactive molecules and fine chemicals and are often used as organocatalysts or as auxiliary ligands in transition metal complexes assisted catalyses. Various kinds of palladium-containing complexes as active catalysts in the C(sp3)-H or C(sp2)-H bond activation have attracted increasing attention because of their accessibility and catalytic efficiencies. This review summarizes the recent developments in this area which employs Pd0-PdIV as versatile catalyst precursors to the syntheses of N-heterocycles. The N-heterocycles, including five- and six-membered rings, are described separately. Furthermore, some of the asymmetric C-H bond activation and mechanistic aspects of these catalytic reactions are discussed.
Evolution of C-H Bond Functionalization from Methane to Methodology
Evolution of C-H Bond Functionalization from Methane to Methodology
http://pubs.acs.org/doi/abs/10.1021/jacs.5b08707
John F. Hartwig
J. Am. Chem. Soc., Just Accepted Manuscript
DOI: 10.1021/jacs.5b08707
Publication Date (Web): November 13, 2015
Copyright © 2015 American Chemical Society
Abstract: This perspective article will present the fundamental principles, the elementary reactions, the initial catalytic systems, and the contemporary catalytic C-H bond functionalization reactions that have converted C-H bond functionalization from a curiosity to a reality for synthetic chemists. A wide range of elementary reactions involving transition metal complexes cleaves C-H bonds at typically unreactive positions. These reactions, coupled with a separate or simultaneous functionalization process lead to products containing new C-C, C-N and C-O bonds. Such reactions were initially studied for the conversion of light alkanes to liquid products, but they have been used (and commercialized in some cases) most often for the synthesis of the more complex structures of natural products, medicinally active compound, and aromatic materials. Such a change in direction of research in C-H bond functionalization is remarkable because the reactions must occur at an unactivated C-H bond over functional groups that are more reactive than the C-H bond toward classical reagents. The scope of reactions that form C-C bonds or install functionality at an un-activated C-H bond will be presented, and the potential future utility of these reactions will be discussed.
http://pubs.acs.org/doi/abs/10.1021/jacs.5b08707
John F. Hartwig
J. Am. Chem. Soc., Just Accepted Manuscript
DOI: 10.1021/jacs.5b08707
Publication Date (Web): November 13, 2015
Copyright © 2015 American Chemical Society
Abstract: This perspective article will present the fundamental principles, the elementary reactions, the initial catalytic systems, and the contemporary catalytic C-H bond functionalization reactions that have converted C-H bond functionalization from a curiosity to a reality for synthetic chemists. A wide range of elementary reactions involving transition metal complexes cleaves C-H bonds at typically unreactive positions. These reactions, coupled with a separate or simultaneous functionalization process lead to products containing new C-C, C-N and C-O bonds. Such reactions were initially studied for the conversion of light alkanes to liquid products, but they have been used (and commercialized in some cases) most often for the synthesis of the more complex structures of natural products, medicinally active compound, and aromatic materials. Such a change in direction of research in C-H bond functionalization is remarkable because the reactions must occur at an unactivated C-H bond over functional groups that are more reactive than the C-H bond toward classical reagents. The scope of reactions that form C-C bonds or install functionality at an un-activated C-H bond will be presented, and the potential future utility of these reactions will be discussed.
Thursday, November 19, 2015
Light-Driven Heterogeneous Reduction of Carbon Dioxide: Photocatalysts and Photoelectrodes
Light-Driven Heterogeneous Reduction of Carbon Dioxide:
Photocatalysts and Photoelectrodes
James L. White,† Maor F. Baruch,† James E. Pander III,† Yuan Hu,† Ivy C. Fortmeyer,† James Eujin Park,† Tao Zhang,† Kuo Liao,† Jing Gu,‡ Yong Yan,‡ Travis W. Shaw,† Esta Abelev,† and Andrew B. Bocarsly*,†
† Department of Chemistry, Princeton University, Princeton, New Jersey 08544, United States ‡ Chemical and Materials Science Center, National Renewable Energy Laboratory, Golden, Colorado 80401, United States
Chemical Reviews: http://pubs.acs.org/doi/pdf/10.1021/acs.chemrev.5b00370
DOI: 10.1021/acs.chemrev.5b00370
Abstract: Although modern photoelectrochemistry is often traced back to 1972 and the report by Honda and Fujishima1 that a TiO2 photoanode in an electrochemical cell caused the splitting of water into O2 and H2 when illuminated, the first report of this type of phenomenon dates back to Becquerel’s studies, published in 1839.2 This makes photoelectrochemistry one of the oldest investigated techniques for the conversion of sunlight into usable energy. Over this time frame, two general types of photoelectrochemical cells have been developed. The first, typified by Honda’s electrochemistry, is focused primarily on the storage of light energy as high-energy chemical products. Initially, this was termed “artificial photosynthesis” and was focused for the most part on splitting water to generate H2 as an environmentally benign fuel. The second type of photoelectrochemical cell utilizes a chemically reversible redox couple that undergoes a redox change of state at the photoelectrode, followed by conversion of the product species back to the reactant at the counter electrode. The net effect of this reaction is a chemically invariant system that generates electricity from light. The initial implementation of the Gratzel cell, which used ̈ a reversible I2/I3 − couple and a dye-sensitized TiO2 photoanode, is an example of this type of system.3 The work under consideration in this paper focuses on the photosynthetic cells and related systems. However, an analysis of these systems, as is more obviously critical to electricity-generating systems, must take into account whether the system is merely catalytic for the reaction of interest or is a system that actually converts light energy into stored chemical energy. Thus, how one parametrizes and evaluates a heterogeneous photoinduced charge transfer process becomes a critical issue that is therefore reviewed in this work.
James L. White,† Maor F. Baruch,† James E. Pander III,† Yuan Hu,† Ivy C. Fortmeyer,† James Eujin Park,† Tao Zhang,† Kuo Liao,† Jing Gu,‡ Yong Yan,‡ Travis W. Shaw,† Esta Abelev,† and Andrew B. Bocarsly*,†
† Department of Chemistry, Princeton University, Princeton, New Jersey 08544, United States ‡ Chemical and Materials Science Center, National Renewable Energy Laboratory, Golden, Colorado 80401, United States
Chemical Reviews: http://pubs.acs.org/doi/pdf/10.1021/acs.chemrev.5b00370
DOI: 10.1021/acs.chemrev.5b00370
Abstract: Although modern photoelectrochemistry is often traced back to 1972 and the report by Honda and Fujishima1 that a TiO2 photoanode in an electrochemical cell caused the splitting of water into O2 and H2 when illuminated, the first report of this type of phenomenon dates back to Becquerel’s studies, published in 1839.2 This makes photoelectrochemistry one of the oldest investigated techniques for the conversion of sunlight into usable energy. Over this time frame, two general types of photoelectrochemical cells have been developed. The first, typified by Honda’s electrochemistry, is focused primarily on the storage of light energy as high-energy chemical products. Initially, this was termed “artificial photosynthesis” and was focused for the most part on splitting water to generate H2 as an environmentally benign fuel. The second type of photoelectrochemical cell utilizes a chemically reversible redox couple that undergoes a redox change of state at the photoelectrode, followed by conversion of the product species back to the reactant at the counter electrode. The net effect of this reaction is a chemically invariant system that generates electricity from light. The initial implementation of the Gratzel cell, which used ̈ a reversible I2/I3 − couple and a dye-sensitized TiO2 photoanode, is an example of this type of system.3 The work under consideration in this paper focuses on the photosynthetic cells and related systems. However, an analysis of these systems, as is more obviously critical to electricity-generating systems, must take into account whether the system is merely catalytic for the reaction of interest or is a system that actually converts light energy into stored chemical energy. Thus, how one parametrizes and evaluates a heterogeneous photoinduced charge transfer process becomes a critical issue that is therefore reviewed in this work.
Neutral Nickel Catalysts for Olefin Homo- and Copolymerization: Relationships between Catalyst Structures and Catalytic Properties
Neutral Nickel Catalysts for Olefin Homo- and Copolymerization:
Relationships between Catalyst Structures and Catalytic Properties
Hongliang Mu,† Li Pan,*,‡ Dongpo Song,§ and Yuesheng Li*,†,‡
† State Key Laboratory of Polymer Physics and Chemistry, Changchun Institute of Applied Chemistry, Chinese Academy of Sciences, Changchun 130022, China ‡ School of Material Science and Engineering, and Collaborative Innovation Center of Chemical Science and Engineering (Tianjin), Tianjin University, Tianjin 300072, China § Department of Polymer Science and Engineering, University of Massachusetts Amherst, 120 Governors Drive, Amherst, Massachusetts 01003, United States
Chemical Reviews: http://pubs.acs.org/doi/pdf/10.1021/cr500370f
DOI: 10.1021/cr500370f
Abstract: As one milestone in the history of polymerization chemistry, the work of Ziegler and Natta has led to the rapid commercialization of transition metal catalysts for the production of high molecular weight polyolefins.1−4 However, the heterogeneous nature of Ziegler−Natta catalytic systems has hindered better understanding of the polymerization mechanisms, resulting in great difficulties in the design of new catalysts. The advance of homogeneous single-site metallocene catalysts together with the discovery of methylaluminoxane (MAO) is a real breakthrough in understanding the relationship between catalyst structure and catalytic behaviors in olefin polymerization. A more precise control over polymer microstructures has been achieved through modification of the metallocene catalytic systems,5−7 which greatly invigorates the development of new single-site catalysts (postmetallocene catalysts) based on both early and late transition metals.8−17
Hongliang Mu,† Li Pan,*,‡ Dongpo Song,§ and Yuesheng Li*,†,‡
† State Key Laboratory of Polymer Physics and Chemistry, Changchun Institute of Applied Chemistry, Chinese Academy of Sciences, Changchun 130022, China ‡ School of Material Science and Engineering, and Collaborative Innovation Center of Chemical Science and Engineering (Tianjin), Tianjin University, Tianjin 300072, China § Department of Polymer Science and Engineering, University of Massachusetts Amherst, 120 Governors Drive, Amherst, Massachusetts 01003, United States
Chemical Reviews: http://pubs.acs.org/doi/pdf/10.1021/cr500370f
DOI: 10.1021/cr500370f
Abstract: As one milestone in the history of polymerization chemistry, the work of Ziegler and Natta has led to the rapid commercialization of transition metal catalysts for the production of high molecular weight polyolefins.1−4 However, the heterogeneous nature of Ziegler−Natta catalytic systems has hindered better understanding of the polymerization mechanisms, resulting in great difficulties in the design of new catalysts. The advance of homogeneous single-site metallocene catalysts together with the discovery of methylaluminoxane (MAO) is a real breakthrough in understanding the relationship between catalyst structure and catalytic behaviors in olefin polymerization. A more precise control over polymer microstructures has been achieved through modification of the metallocene catalytic systems,5−7 which greatly invigorates the development of new single-site catalysts (postmetallocene catalysts) based on both early and late transition metals.8−17
Electrochemical Synthesis of Photoelectrodes and Catalysts for Use in Solar Water Splitting
Electrochemical Synthesis of Photoelectrodes and Catalysts for Use
in Solar Water Splitting
Donghyeon Kang, Tae Woo Kim, Stephen R. Kubota, Allison C. Cardiel, Hyun Gil Cha, and Kyoung-Shin Choi*
Department of Chemistry, University of Wisconsin-Madison, Madison, Wisconsin 53706, United States
Chemical Reviews: http://pubs.acs.org/doi/pdf/10.1021/acs.chemrev.5b00498
DOI: 10.1021/acs.chemrev.5b00498
Abstract: This review focuses on introducing and explaining electrodepostion mechanisms and electrodeposition-based synthesis strategies used for the production of catalysts and semiconductor electrodes for use in water-splitting photoelectrochemical cells (PECs). It is composed of three main sections: electrochemical synthesis of hydrogen evolution catalysts, oxygen evolution catalysts, and semiconductor electrodes. The semiconductor section is divided into two parts: photoanodes and photocathodes. Photoanodes include n-type semiconductor electrodes that can perform water oxidation to O2 using photogenerated holes, while photocathodes include p-type semiconductor electrodes that can reduce water to H2 using photoexcited electrons. For each material type, deposition mechanisms were reviewed first followed by a brief discussion on its properties relevant to electrochemical and photoelectrochemical water splitting. Electrodeposition or electrochemical synthesis is an ideal method to produce individual components and integrated systems for PECs due to its various intrinsic advantages. This review will serve as a good resource or guideline for researchers who are currently utilizing electrochemical synthesis as well as for those who are interested in beginning to employ electrochemical synthesis for the construction of more efficient PECs.
Donghyeon Kang, Tae Woo Kim, Stephen R. Kubota, Allison C. Cardiel, Hyun Gil Cha, and Kyoung-Shin Choi*
Department of Chemistry, University of Wisconsin-Madison, Madison, Wisconsin 53706, United States
Chemical Reviews: http://pubs.acs.org/doi/pdf/10.1021/acs.chemrev.5b00498
DOI: 10.1021/acs.chemrev.5b00498
Abstract: This review focuses on introducing and explaining electrodepostion mechanisms and electrodeposition-based synthesis strategies used for the production of catalysts and semiconductor electrodes for use in water-splitting photoelectrochemical cells (PECs). It is composed of three main sections: electrochemical synthesis of hydrogen evolution catalysts, oxygen evolution catalysts, and semiconductor electrodes. The semiconductor section is divided into two parts: photoanodes and photocathodes. Photoanodes include n-type semiconductor electrodes that can perform water oxidation to O2 using photogenerated holes, while photocathodes include p-type semiconductor electrodes that can reduce water to H2 using photoexcited electrons. For each material type, deposition mechanisms were reviewed first followed by a brief discussion on its properties relevant to electrochemical and photoelectrochemical water splitting. Electrodeposition or electrochemical synthesis is an ideal method to produce individual components and integrated systems for PECs due to its various intrinsic advantages. This review will serve as a good resource or guideline for researchers who are currently utilizing electrochemical synthesis as well as for those who are interested in beginning to employ electrochemical synthesis for the construction of more efficient PECs.
Oxidative Coupling between Two Hydrocarbons: An Update of Recent C–H Functionalizations
Oxidative Coupling between Two Hydrocarbons: An Update of Recent C-H Functionalizations
Chao Liu,† Jiwen Yuan,† Meng Gao,‡ Shan Tang,† Wu Li,† Renyi Shi,† and Aiwen Lei*,†,‡
† College of Chemistry and Molecular Sciences, Institute for Advanced Studies (IAS), Wuhan University, Wuhan 430072, People’s Republic of China ‡ National Research Center for Carbohydrate Synthesis, Jiangxi Normal University, Nanchang, Jiangxi 330022, People’s Republic of China
Chemical Reviews; http://pubs.acs.org/doi/pdf/10.1021/cr500431s
DOI: 10.1021/cr500431s
Abstract: Somewhat comprehensive review of recent C-H functionalizations. Note this was received August 7, 2014.
Chao Liu,† Jiwen Yuan,† Meng Gao,‡ Shan Tang,† Wu Li,† Renyi Shi,† and Aiwen Lei*,†,‡
† College of Chemistry and Molecular Sciences, Institute for Advanced Studies (IAS), Wuhan University, Wuhan 430072, People’s Republic of China ‡ National Research Center for Carbohydrate Synthesis, Jiangxi Normal University, Nanchang, Jiangxi 330022, People’s Republic of China
Chemical Reviews; http://pubs.acs.org/doi/pdf/10.1021/cr500431s
DOI: 10.1021/cr500431s
Abstract: Somewhat comprehensive review of recent C-H functionalizations. Note this was received August 7, 2014.
Tuesday, November 17, 2015
Removing Homogeneous Catalysts from Reaction Mixtures for Product Isolation
Metathesis Catalyst Removal Techniques
Monday, November 16, 2015
Orthogonal tandem catalysis
http://www.nature.com/nchem/journal/v7/n6/full/nchem.2262.html
Orthogonal tandem catalysis
- Nature Chemistry 7, 477–482 doi:10.1038/nchem.2262
Abstract
Tandem catalysis is a growing field that is beginning to yield important scientific and technological advances toward new and more efficient catalytic processes. 'One-pot' tandem reactions, where multiple catalysts and reagents, combined in a single reaction vessel undergo a sequence of precisely staged catalytic steps, are highly attractive from the standpoint of reducing both waste and time. Orthogonal tandem catalysis is a subset of one-pot reactions in which more than one catalyst is used to promote two or more mechanistically distinct reaction steps. This Perspective summarizes and analyses some of the recent developments and successes in orthogonal tandem catalysis, with particular focus on recent strategies to address catalyst incompatibility. We also highlight the concept of thermodynamic leveraging by coupling multiple catalyst cycles to effect challenging transformations not observed in single-step processes, and to encourage application of this technique to energetically unfavourable or demanding reactions.Friday, November 13, 2015
Iridium(III) Bis-Pyridine-2-Sulfonamide Complexes as Efficient and Durable Catalysts for Homogeneous Water Oxidation
Iridium(III) Bis-Pyridine-2-Sulfonamide Complexes as Efficient and
Durable Catalysts for Homogeneous Water Oxidation
Mo Li,† Kazutake Takada,‡ Jonas I. Goldsmith,*,§ and Stefan Bernhard*,†
† Department of Chemistry, Carnegie Mellon University, Pittsburgh, Pennsylvania 15213, United States ‡ Department of Materials Science and Engineering, Graduate School of Engineering, Nagoya Institute of Technology, Gokiso-cho, Showa-ku, Nagoya 466-8555, Japan § Department of Chemistry, Bryn Mawr College, Bryn Mawr, Pennsylvania 19010, United States
Inorganic Chemistry; http://pubs.acs.org/doi/pdf/10.1021/acs.inorgchem.5b01709
Abstract:
A family of tetradentate bis(pyridine-2-sulfonamide) (bpsa) compounds was synthesized as a ligand platform for designing resilient and electronically tunable catalysts capable of performing water oxidation catalysis and other processes in highly oxidizing environments. These wrap-around ligands were coordinated to Ir(III) octahedrally, forming an anionic complex with chloride ions bound to the two remaining coordination sites. NMR spectroscopy documented that the more rigid ligand frameworks--[Ir(bpsa-Cy)Cl2] − and [Ir(bpsa-Ph)Cl2] −produced C1-symmetric complexes, while the complex with the more flexible ethylene linker in [Ir(bpsaen)Cl2] − displays C2 symmetry. Their electronic structure was explored with DFT calculations and cyclic voltammetry in nonaqueous environments, which unveiled highly reversible Ir(III)/Ir(IV) redox processes and more complex, irreversible reduction chemistry. Addition of water to the electrolyte revealed the ability of these complexes to catalyze the water oxidation reaction efficiently. Electrochemical quartz crystal microbalance studies confirmed that a molecular species is responsible for the observed electrocatalytic behavior and ruled out the formation of active IrOx. The electrochemical studies were complemented by work on chemically driven water oxidation, where the catalytic activity of the iridium complexes was studied upon exposure to ceric ammonium nitrate, a strong, one-electron oxidant. Variation of the catalyst concentrations helped to illuminate the kinetics of these water oxidation processes and highlighted the robustness of these systems. Stable performance for over 10 days with thousands of catalyst turnovers was observed with the C1-symmetric catalysts. Dynamic light scattering experiments ascertained that a molecular species is responsible for the catalytic activity and excluded the formation of IrOx particles.
TOC:
Mo Li,† Kazutake Takada,‡ Jonas I. Goldsmith,*,§ and Stefan Bernhard*,†
† Department of Chemistry, Carnegie Mellon University, Pittsburgh, Pennsylvania 15213, United States ‡ Department of Materials Science and Engineering, Graduate School of Engineering, Nagoya Institute of Technology, Gokiso-cho, Showa-ku, Nagoya 466-8555, Japan § Department of Chemistry, Bryn Mawr College, Bryn Mawr, Pennsylvania 19010, United States
Inorganic Chemistry; http://pubs.acs.org/doi/pdf/10.1021/acs.inorgchem.5b01709
Abstract:
A family of tetradentate bis(pyridine-2-sulfonamide) (bpsa) compounds was synthesized as a ligand platform for designing resilient and electronically tunable catalysts capable of performing water oxidation catalysis and other processes in highly oxidizing environments. These wrap-around ligands were coordinated to Ir(III) octahedrally, forming an anionic complex with chloride ions bound to the two remaining coordination sites. NMR spectroscopy documented that the more rigid ligand frameworks--[Ir(bpsa-Cy)Cl2] − and [Ir(bpsa-Ph)Cl2] −produced C1-symmetric complexes, while the complex with the more flexible ethylene linker in [Ir(bpsaen)Cl2] − displays C2 symmetry. Their electronic structure was explored with DFT calculations and cyclic voltammetry in nonaqueous environments, which unveiled highly reversible Ir(III)/Ir(IV) redox processes and more complex, irreversible reduction chemistry. Addition of water to the electrolyte revealed the ability of these complexes to catalyze the water oxidation reaction efficiently. Electrochemical quartz crystal microbalance studies confirmed that a molecular species is responsible for the observed electrocatalytic behavior and ruled out the formation of active IrOx. The electrochemical studies were complemented by work on chemically driven water oxidation, where the catalytic activity of the iridium complexes was studied upon exposure to ceric ammonium nitrate, a strong, one-electron oxidant. Variation of the catalyst concentrations helped to illuminate the kinetics of these water oxidation processes and highlighted the robustness of these systems. Stable performance for over 10 days with thousands of catalyst turnovers was observed with the C1-symmetric catalysts. Dynamic light scattering experiments ascertained that a molecular species is responsible for the catalytic activity and excluded the formation of IrOx particles.
TOC:
Love at second sight for CO2 and H2
Science
6 November 2015:
Vol. 350 no. 6261 pp. 629-630
DOI: 10.1126/science.aac7997
Vol. 350 no. 6261 pp. 629-630
DOI: 10.1126/science.aac7997
Love at second sight for CO2 and H2 in organic synthesis
+ Author Affiliations
Many romantic comedies are based on a
couple with opposite personalities who fall in love, lose track of each
other, and reunite
against all odds, most often with the help of a
mediator. This cinematic scenario recaps the recent progress in bringing
together
two molecular actors, carbon dioxide (CO2) and hydrogen (H2), with the help of homogeneous catalysts in solution. Of the pair, CO2 is the reluctant partner; it is thermodynamically very stable and kinetically inert in typical organic syntheses. However,
an energetic partner such as H2 can bring out its reactivity if their combination is properly directed. Organometallic catalysts have recently opened new
possibilities to merge this odd couple of CO2 and H2 and, with the support of helpful in-laws (co-reactants), to establish synthetic methods for sustainable chemical processes
across the chemical value chain.
Thursday, November 12, 2015
Bulky N‑Phosphino-Functionalized N‑Heterocyclic Carbene Ligands: Synthesis, Ruthenium Coordination Chemistry, and Ruthenium Alkylidene Complexes for Olefin Metathesis
Bulky N‑Phosphino-Functionalized N‑Heterocyclic Carbene Ligands:
Synthesis, Ruthenium Coordination Chemistry, and Ruthenium
Alkylidene Complexes for Olefin Metathesis
Bing Wu, Kathryn M. Gramigna, Mark W. Bezpalko, Bruce M. Foxman, and Christine M. Thomas*
Department of Chemistry, Brandeis University, 415 South Street, Waltham, Massachusetts 02454, United States
Inorganic Chemistry; http://pubs.acs.org/doi/pdf/10.1021/acs.inorgchem.5b00513
Abstract:
Ruthenium chemistry and applications in catalytic olefin metathesis based on N-phosphino-functionalized N-heterocyclic carbene ligands (NHCPs) are presented. Alkyl NHCP Ru coordination chemistry is described, and access to several potential synthetic precursors for ruthenium alkylidene complexes is outlined, incorporating both trimethylsilyl and phenyl alkylidenes. The Ru alkylidene complexes are evaluated as potential olefin metathesis catalysts and were shown to behave in a latent fashion. They displayed catalytic activity at elevated temperatures for both ring closing metathesis and ring opening metathesis polymerization.
TOC:
Bing Wu, Kathryn M. Gramigna, Mark W. Bezpalko, Bruce M. Foxman, and Christine M. Thomas*
Department of Chemistry, Brandeis University, 415 South Street, Waltham, Massachusetts 02454, United States
Inorganic Chemistry; http://pubs.acs.org/doi/pdf/10.1021/acs.inorgchem.5b00513
Abstract:
Ruthenium chemistry and applications in catalytic olefin metathesis based on N-phosphino-functionalized N-heterocyclic carbene ligands (NHCPs) are presented. Alkyl NHCP Ru coordination chemistry is described, and access to several potential synthetic precursors for ruthenium alkylidene complexes is outlined, incorporating both trimethylsilyl and phenyl alkylidenes. The Ru alkylidene complexes are evaluated as potential olefin metathesis catalysts and were shown to behave in a latent fashion. They displayed catalytic activity at elevated temperatures for both ring closing metathesis and ring opening metathesis polymerization.
TOC:
Hypervalent Iodine-Mediated Fluorination of Styrene Derivatives: Stoichiometric and Catalytic Transformation to 2,2- Difluoroethylarenes
Hypervalent Iodine-Mediated Fluorination of Styrene Derivatives:
Stoichiometric and Catalytic Transformation to 2,2-
Difluoroethylarenes
Tsugio Kitamura,* Kensuke Muta, and Juzo Oyamada Department of Chemistry and Applied Chemistry, Graduate School of Science and Engineering, Saga University, Hojo-machi, Saga 840-8502, Japan
Journal of Organic Chemistry http://pubs.acs.org/doi/pdf/10.1021/acs.joc.5b01929
Abstract:
Fluorination of styrene derivatives with a reagent system composed of μ-oxo-bis[trifluoroacetato(phenyl)iodine] and a pyridine·HF complex gave the corresponding (2,2-difluoroethyl)arenes in good yields. Similarly, the reagent of PhI(OCOCF3)2 and the pyridine·HF complex acted as a fluorinating agent for styrene derivatives. The fluorination of styrene derivatives with the pyridine· HF complex underwent under catalytic conditions using 4-iodotoluene as a catalyst and m-CPBA as a terminal oxidant.
TOC:
Tsugio Kitamura,* Kensuke Muta, and Juzo Oyamada Department of Chemistry and Applied Chemistry, Graduate School of Science and Engineering, Saga University, Hojo-machi, Saga 840-8502, Japan
Journal of Organic Chemistry http://pubs.acs.org/doi/pdf/10.1021/acs.joc.5b01929
Abstract:
Fluorination of styrene derivatives with a reagent system composed of μ-oxo-bis[trifluoroacetato(phenyl)iodine] and a pyridine·HF complex gave the corresponding (2,2-difluoroethyl)arenes in good yields. Similarly, the reagent of PhI(OCOCF3)2 and the pyridine·HF complex acted as a fluorinating agent for styrene derivatives. The fluorination of styrene derivatives with the pyridine· HF complex underwent under catalytic conditions using 4-iodotoluene as a catalyst and m-CPBA as a terminal oxidant.
TOC:
Contrasting Mechanisms and Reactivity of Tl(III), Hg(II), and Co(III) for Alkane C−H Functionalization
Organometallics ASAP
Contrasting Mechanisms and Reactivity of Tl(III), Hg(II), and Co(III) for Alkane C−H Functionalization
Samantha J. Gustafson,† Jack T. Fuller, III,† Deepa Devarajan,† Justin Snyder,† Roy A. Periana,‡ Brian G. Hashiguchi,‡ Michael M. Konnick,‡ and Daniel H. Ess*,†
† Department of Chemistry and Biochemistry, Brigham Young University, Provo, Utah 84602, United States
‡ Department of Chemistry, The Scripps Research Institute, Jupiter, Florida 33458, United States
Abstract: Activation and functionalization of alkane C–H bonds has historically been dominated by transition-metal complexes. Light alkanes can also be partially oxidized by sixth-row main-group compounds, such as TlIII(TFA)3 (TFA = trifluoroacetate). Here we present density-functional calculations which demonstrate that TlIII(TFA)3 oxidizes alkanes by closed-shell C–H activation and Tl–alkyl functionalization mechanisms. The discovery of a C–H activation pathway is surprising, because TlIII often oxidizes arene C–H bonds through an electron transfer mechanism and the transition-metal complex CoIII(TFA)3, with similar oxidation state and ligand coordination, oxidizes alkanes via an open-shell radical mechanism. Comparison of TlIII(TFA)3to the transition-metal analogue IrIII(TFA)3 reveals that key to TlIII oxidation of alkanes is a moderate barrier for C–H bond activation that is lower in energy than open-shell pathways and a subsequent metal–alkyl functionalization reaction step with a very low barrier. Our calculations suggest that the high-spin ground state of CoIII(TFA)3 provides a low-energy open-shell decarboxylation pathway that leads to radical oxidation of alkanes, which is not available for the d10 TlIII(TFA)3 complex. The C–H activation pathway and transition state model provide a straightforward explanation for why TlIII(TFA)3 promotes alkane C–H bond activation but HgII(TFA)2 does not.
Progress in Synthesis of Highly Active and Stable Nickel-Based Catalysts for Carbon Dioxide Reforming of Methane
Prof. Sibudjing Kawi, Dr. Yasotha Kathiraser, Dr. Jun Ni, Dr. Usman Oemar, Dr. Ziwei Li, Dr. Eng Toon Saw
http://onlinelibrary.wiley.com/doi/10.1002/cssc.201500390/full
Abstract
In recent decades, rising anthropogenic greenhouse gas emissions (mainly CO2 and CH4) have increased alarm due to escalating effects of global warming. The dry carbon dioxide reforming of methane (DRM) reaction is a sustainable way to utilize these notorious greenhouse gases. This paper presents a review of recent progress in the development of nickel-based catalysts for the DRM reaction. The enviable low cost and wide availability of nickel compared with noble metals is the main reason for persistent research efforts in optimizing the synthesis of nickel-based catalysts. Important catalyst features for the rational design of a coke-resistant nickel-based nanocatalyst for the DRM reaction are also discussed. In addition, several innovative developments based on salient features for the stabilization of nickel nanocatalysts through various means (which include functionalization with precursors, synthesis by plasma treatment, stabilization/confinement on mesoporous/microporous/carbon supports, and the formation of metal oxides) are highlighted. The final part of this review covers major issues and proposed improvement strategies pertaining to the rational design of nickel-based catalysts with high activity and stability for the DRM reaction.http://onlinelibrary.wiley.com/doi/10.1002/cssc.201500390/full
Efficient Light-Driven Water Oxidation Catalysis by Dinuclear Ruthenium Complexes
Dr. Serena Berardi, Dr. Laia Francas, Sven Neudeck, Dr. Somnath Maji, Dr. Jordi Benet-Buchholz, Prof. Dr. Franc Meyer, Prof. Dr. Antoni Llobet
Abstract
Mastering the light-induced four-electron oxidation of water to molecular oxygen is a key step towards the achievement of overall water splitting to produce alternative solar fuels. In this work, we report two rugged molecular pyrazolate-based diruthenium complexes that efficiently catalyze visible-light-driven water oxidation. These complexes were fully characterized both in the solid state (by X-ray diffraction analysis) and in solution (spectroscopically and electrochemically). Benchmark performances for homogeneous oxygen production have been obtained for both catalysts in the presence of a photosensitizer and a sacrificial electron acceptor at pH 7, and a turnover frequency of up to 11.1 s−1 and a turnover number of 5300 were obtained after three successive catalytic runs. Under the same experimental conditions with the same setup, the pyrazolate-based diruthenium complexes outperform other well-known water oxidation catalysts owing to both electrochemical and mechanistic aspects.http://onlinelibrary.wiley.com/doi/10.1002/cssc.201500798/full
Phase transition-induced band edge engineering of BiVO4 to split pure water under visible light
Won Jun Joa,1,Hyun Joon Kangb,1,Ki-Jeong Kongc, Yun Seog Leed, Hunmin Parkb, Younghye Leeb, Tonio Buonassisid, Karen K. Gleasona, and Jae Sung Leee,2
aDepartment of Chemical Engineering, Massachusetts Institute of Technology, Cambridge, MA 02139; bDepartment of Chemical Engineering, Pohang University of Science and Technology, Pohang 790-784, Korea;cKorea Institute of Chemical Technology, Daejeon 305-343, Korea; dDepartment of Mechanical Engineering, Massachusetts Institute of Technology, Cambridge, MA 02139;eSchool of Energy and Chemical Engineering, Ulsan National Institute of Science and Technology, Ulsan 689-798, Korea
Abstract
Through phase transition-induced band edge engineering by dual doping with In and Mo, a new greenish BiVO4 (Bi1-XInXV1-XMoXO4) is developed that has a larger band gap energy than the usual yellow scheelite monoclinic BiVO4 as well as a higher (more negative) conduction band than H+/H2 potential [0 VRHE (reversible hydrogen electrode) at pH 7]. Hence, it can extract H2 from pure water by visible light-driven overall water splitting without using any sacrificial reagents. The density functional theory calculation indicates that In3+/Mo6+ dual doping triggers partial phase transformation from pure monoclinic BiVO4 to a mixture of monoclinic BiVO4 and tetragonal BiVO4, which sequentially leads to unit cell volume growth, compressive lattice strain increase, conduction band edge uplift, and band gap widening.
http://www.pnas.org/content/112/45/13774.full
Wednesday, November 11, 2015
Tutorial on Oxidative Addition
By Jay A. Labinger*
ABSTRACT: This tutorial introduces oxidative addition as a reactivity pattern and organizing principle for organometallic chemistry. The history, characteristics, and scope of oxidative addition are briefly surveyed, followed by a detailed examination of the variety of mechanisms found for the oxidative addition of alkyl halides and their relevance to practical applications.
http://pubs.acs.org/doi/pdf/10.1021/acs.organomet.5b00565
By Jay A. Labinger*
ABSTRACT: This tutorial introduces oxidative addition as a reactivity pattern and organizing principle for organometallic chemistry. The history, characteristics, and scope of oxidative addition are briefly surveyed, followed by a detailed examination of the variety of mechanisms found for the oxidative addition of alkyl halides and their relevance to practical applications.
http://pubs.acs.org/doi/pdf/10.1021/acs.organomet.5b00565
Monday, November 9, 2015
Determining the "Best Catalyst" Depends on the Method of Catalyst Evaluation
http://pubs.acs.org/doi/abs/10.1021/jacs.5b05093
The “Best Catalyst” for Water Oxidation Depends on the Oxidation Method Employed: A Case Study of Manganese Oxides
Department
of Chemistry, University of Wisconsin—Madison, 1101 University Avenue, Madison, Wisconsin 53706, United States
J. Am. Chem. Soc., 2015, 137 (26), pp 8384–8387
DOI: 10.1021/jacs.5b05093
Abstract

Manganese oxides are a highly promising class of water-oxidation catalysts (WOCs), but the optimal MnOx formulation or polymorph is not clear from previous reports in the literature. A complication not limited to MnOx-based
WOCs is that such catalysts are routinely evaluated by different
methods, ranging from the use of a chemical oxidant such as Ce4+, photoactive mediators such as [Ru(bpy)3]2+, or electrochemical techniques. Here, we report a systematic study of nine crystalline MnOx
materials as WOCs and show that the identity of the “best” catalyst
changes, depending on the oxidation method used to probe the catalytic
activity.
Wednesday, November 4, 2015
Highly Efficient Gold(I)-Catalyzed Regio- and Stereoselective Hydrocarboxylation of Internal Alkynes
http://pubs.acs.org/doi/abs/10.1021/acscatal.5b02090
Stéphanie Dupuy†, Danila Gasperini†, and Steven P. Nolan*‡
† EaStCHEM School of Chemistry, University of St. Andrews, North Haugh, St. Andrews, Fife KY16 9ST,U.K.
‡ Chemistry Department, College of Science, King Saud University, Riyadh 11451, Saudi Arabia
ACS Catal., 2015, 5, pp 6918–6921
DOI: 10.1021/acscatal.5b02090
Publication Date (Web): October 12, 2015
Abstract:
We report the highly efficient gold-catalyzed hydrocarboxylation of internal alkynes that operates under solvent- and silver-free conditions. This new, simple, and eco-friendly protocol allows for the synthesis of a wide variety of functionalized aryl and alkyl enol esters in high yields, with Z-stereospecificity and good regioselectivities and without the requirement for purification by chromatography. This process represents an expedient, operationally simple method for the synthesis of enol esters.

Keywords: gold catalysis; carboxylic acids; internal alkynes; N-heterocyclic carbenes;cooperativity
‡ Chemistry Department, College of Science, King Saud University, Riyadh 11451, Saudi Arabia
ACS Catal., 2015, 5, pp 6918–6921
DOI: 10.1021/acscatal.5b02090
Publication Date (Web): October 12, 2015
Abstract:
We report the highly efficient gold-catalyzed hydrocarboxylation of internal alkynes that operates under solvent- and silver-free conditions. This new, simple, and eco-friendly protocol allows for the synthesis of a wide variety of functionalized aryl and alkyl enol esters in high yields, with Z-stereospecificity and good regioselectivities and without the requirement for purification by chromatography. This process represents an expedient, operationally simple method for the synthesis of enol esters.

Keywords: gold catalysis; carboxylic acids; internal alkynes; N-heterocyclic carbenes;cooperativity
Principles and Applications of Enantioselective Hydroformylation of Terminal Disubstituted Alkenes
http://pubs.acs.org/doi/abs/10.1021/acscatal.5b01300
Yuchao Deng†‡, Hui Wang†, Yuhan Sun†, and Xiao Wang*†§
† CAS Key Lab of Low-Carbon Conversion Science and Engineering, Shanghai Advanced Research Institute, Chinese Academy of Sciences, 100 Haike Road, Pudong, Shanghai 201210, People’s Republic of China
‡ School of Physical Science and Technology, Shanghai Tech University, 100 Haike Road, Pudong, Shanghai, 201210, People’s Republic of China
§ Harvard NeuroDiscovery Center, Harvard Medical School and Brigham & Women’s Hospital, 65 Landsdowne Street, Cambridge, Massachusetts 02139, United States
Yuchao Deng†‡, Hui Wang†, Yuhan Sun†, and Xiao Wang*†§
† CAS Key Lab of Low-Carbon Conversion Science and Engineering, Shanghai Advanced Research Institute, Chinese Academy of Sciences, 100 Haike Road, Pudong, Shanghai 201210, People’s Republic of China
‡ School of Physical Science and Technology, Shanghai Tech University, 100 Haike Road, Pudong, Shanghai, 201210, People’s Republic of China
§ Harvard NeuroDiscovery Center, Harvard Medical School and Brigham & Women’s Hospital, 65 Landsdowne Street, Cambridge, Massachusetts 02139, United States
Closing the loop: captured CO2 as a feedstock in the chemical industry
Alexander Otto, Thomas Grube, Sebastian Schieben, and Detlef Stolten
Energy Environ. Sci. 2015, 8, 3283-3297
Abstract: The utilization of ‘captured’ CO2 as a feedstock in the chemical industry for the synthesis of certain chemical products offers an option for preventing several million tons of CO2 emissions each year while increasing independence from fossil fuels. For this reason, interest is increasing in the feasibility of deploying captured CO2 in this manner. Numerous scientific publications describe laboratory experiments in which CO2 has been successfully used as a feedstock for the synthesis of various chemical products. However, many of these publications have focused on the feasibility of syntheses without considering the ancillary benefits of CO2 emissions reduction if the CO2 is sourced from effluent or the potential profitability of this process. Evaluating these environmental and economic benefits is important for promoting the further development of benign CO2 applications. Given the multitude of CO2 utilization reactions in the laboratory context, an initial assessment must be undertaken to identify those which have the most potential for future technical exploration and development. To achieve this, 123 reactions from the literature were identified and evaluated with the help of selection criteria specifically developed for this project. These criteria incorporate both the quantitative potential of reducing CO2 and possible economic benefits of these syntheses. The selected reactions are divided into bulk and fine chemicals. Of the bulk chemicals, formic acid, oxalic acid, formaldehyde, methanol, urea and dimethyl ether, and of the fine chemicals, methylurethane, 3-oxo-pentanedioic acid, 2-imidazolidinone, ethylurethane, 2-oxazolidone and isopropyl isocyanate, mostly fulfil the selection criteria in each category.
Tuesday, November 3, 2015
Kinetics and Mechanism of the Chlorate−Bromide Reaction
Kinetics and Mechanism of the Chlorate−Bromide Reaction
Rafaela T. P. Sant’Anna and Roberto B. Faria*
Instituto de Química, Universidade Federal do Rio de Janeiro, Av. Athos da Silveira Ramos 149, CT, Bloco A, 21941-611 Rio de Janeiro, RJ Brazil
Inorg. Chem. 2015, 54, 10415-10421.
http://pubs.acs.org/doi/pdf/10.1021/acs.inorgchem.5b01857
Abstract:
The chlorate−bromide reaction, ClO3 − + 6Br− + 6H+ → 3Br2 + Cl− + 3H2O, was followed at the Br3 −/Br2 isosbestic point (446 nm). A fifthorder rate law was found: 1 /3 d[Br2]/dt = k[ClO3 −][Br−][H+ ] 3 (k = 5.10 × 10−6 s −1 L4 mol−4 ) at 25 °C and I = 2.4 mol L−1 . At high bromide concentrations, the bromide order becomes close to zero, indicating a saturation profile on bromide concentration, similar to the chloride saturation profile observed in the chlorate−chloride reaction. A mechanism is proposed that considers the formation of the intermediate BrOClO2 2−, similar to the intermediate ClOClO2 2− proposed in the mechanism of the chlorate−chloride reaction.
TOC:
Rafaela T. P. Sant’Anna and Roberto B. Faria*
Instituto de Química, Universidade Federal do Rio de Janeiro, Av. Athos da Silveira Ramos 149, CT, Bloco A, 21941-611 Rio de Janeiro, RJ Brazil
Inorg. Chem. 2015, 54, 10415-10421.
http://pubs.acs.org/doi/pdf/10.1021/acs.inorgchem.5b01857
Abstract:
The chlorate−bromide reaction, ClO3 − + 6Br− + 6H+ → 3Br2 + Cl− + 3H2O, was followed at the Br3 −/Br2 isosbestic point (446 nm). A fifthorder rate law was found: 1 /3 d[Br2]/dt = k[ClO3 −][Br−][H+ ] 3 (k = 5.10 × 10−6 s −1 L4 mol−4 ) at 25 °C and I = 2.4 mol L−1 . At high bromide concentrations, the bromide order becomes close to zero, indicating a saturation profile on bromide concentration, similar to the chloride saturation profile observed in the chlorate−chloride reaction. A mechanism is proposed that considers the formation of the intermediate BrOClO2 2−, similar to the intermediate ClOClO2 2− proposed in the mechanism of the chlorate−chloride reaction.
TOC:
Influence of Elemental Iodine on Imidazolium-Based Ionic Liquids: Solution and Solid-State Effects
Influence of Elemental Iodine on Imidazolium-Based Ionic Liquids:
Solution and Solid-State Effects
Zhaofu Fei, Felix D. Bobbink, Emilia Pa ́ unescu, Rosario Scopelliti, and Paul J. Dyson*
Institut des Sciences et Ingenierie Chimiques, Ecole Polytechnique Fe ́ dé rale de Lausanne (EPFL), CH-1015 Lausanne, Switzerland
Inorg. Chem. 2015, 54, 10504-10512.
http://pubs.acs.org/doi/pdf/10.1021/acs.inorgchem.5b02021
Abstract:
Ionic liquids doped with I2, usually resulting in the formation of polyiodide anions, are extensively used as electrolytes and in iodination reactions. Herein, NMR spectroscopy and single-crystal X-ray diffraction were used to rationalize the structures of imidazolium-based polyiodide ionic liquids in the liquid and solid states. Combined, these studies show that extensive interactions between the imidazolium cation and the resulting polyiodide anion are present, which have the net effect of lengthening, polarizing, and weakening the I−I bonds in the anion. This bond weakening rationalizes the high conductivity and reactivity of ionic liquids doped with I2.
TOC:
Zhaofu Fei, Felix D. Bobbink, Emilia Pa ́ unescu, Rosario Scopelliti, and Paul J. Dyson*
Institut des Sciences et Ingenierie Chimiques, Ecole Polytechnique Fe ́ dé rale de Lausanne (EPFL), CH-1015 Lausanne, Switzerland
Inorg. Chem. 2015, 54, 10504-10512.
http://pubs.acs.org/doi/pdf/10.1021/acs.inorgchem.5b02021
Abstract:
Ionic liquids doped with I2, usually resulting in the formation of polyiodide anions, are extensively used as electrolytes and in iodination reactions. Herein, NMR spectroscopy and single-crystal X-ray diffraction were used to rationalize the structures of imidazolium-based polyiodide ionic liquids in the liquid and solid states. Combined, these studies show that extensive interactions between the imidazolium cation and the resulting polyiodide anion are present, which have the net effect of lengthening, polarizing, and weakening the I−I bonds in the anion. This bond weakening rationalizes the high conductivity and reactivity of ionic liquids doped with I2.
TOC:
From proton transferred to cyclometalated platinum(IV) complex: Crystal structure and biological activity
http://www.sciencedirect.com/science/article/pii/S0022328X1530125X
- a Department of Chemistry, North Tehran Branch, Islamic Azad University, Tehran 19585-936, Iran
- b Department of Chemistry, Shahid Beheshti University, G. C., Evin, Tehran 19839-63113, Iran
- c Department of Toxicology & Pharmacology, University of Tehran Medical Sciences, Tehran 14155-6451, Iran
- doi:10.1016/j.jorganchem.2015.08.023
- JOMC 2015, 800, 30-37
- Abstract: A new proton transfer complex of [6,6′-dmbipy.H]2[PtCl6] (1) was prepared from the reaction of H2PtCl6.6H2O with 6,6′-dimethyl-2,2'-bipyridine (6,6′-dmbipy) in CH3CN at room temperature. The cyclometalated complex of mer-[Pt(6,6′-dmbipy-κ2N,C)Cl3(DMF-κO)] (2) was prepared from the recrystallization of complex 1, in a mixture of DMF/DMSO/H2O (6:2:1) at 60 °C, in which the first C–H bond activation in a bipyridine ring with Pt(IV) ion was observed. In vitro cytotoxicity against four cultures: NIH-3T3, Caco-2, HT-29 and T47D were studied using MTT assays. Interestingly, compound 2 is more potent in killing a colon cancer cell line than cisplatin, since it exhibits extensively less toxicity on normal cells. Both complexes were characterized by elemental analysis, FT-IR, 1H NMR, UV–Vis spectra and X-ray crystallography.
Subscribe to:
Posts (Atom)