Furfural: a renewable and versatile platform molecule for the synthesis of chemicals and fuels
R. Mariscal, P. Maireles-Torres, M. Ojeda, I. Sádabaa and M. López Granados*
Energy Environ. Sci., 2016, Advance Article
DOI: 10.1039/C5EE02666K
http://pubs.rsc.org/en/content/articlelanding/2016/ee/c5ee02666k#!divAbstract
Received 30 Aug 2015, Accepted 11 Jan 2016
First published online 11 Jan 2016
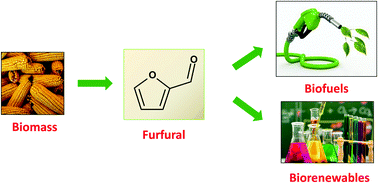
The production of future transportation fuels and chemicals requires the deployment of new catalytic processes that transform biomass into valuable products under competitive conditions. Furfural has been identified as one of the most promising chemical platforms directly derived from biomass. With an annual production close to 300 kTon, furfural is currently a commodity chemical, and the technology for its production is largely established. The aim of this review is to discuss the most relevant chemical routes for converting furfural to chemicals, biofuels, and additives. This review focuses not only on industrially produced chemicals derived from furfural, but also on other not yet commercialised products that have a high potential for commercialisation as commodities. Other chemicals that are currently produced from oil but can also be derived from furfural are also reviewed. The chemical and engineering aspects such as the reaction conditions and mechanisms, as well as the main achievements and the challenges still to come in the pursuit of advancing the furfural-based industry, are highlighted.
Tuesday, March 29, 2016
Wednesday, March 16, 2016
Recent Advances in Process Chemistry
http://pubs.acs.org/doi/pdf/10.1021/acs.oprd.6b00012
Lots of good stuff in this one, below is one example.

Lots of good stuff in this one, below is one example.
Synthesis of 2,3,6-Trisubstituted Pyridines from Isoxazolinones

Substituted
pyridines are an important class of organic compounds and ubiquitous in
the chemistry world. Various methodologies have been developed to
construct substituted pyridine derivatives. Recently, René Peters and
co-workers developed a regioselective Pd-catalyzed synthesis of
2,3,6-trisubstituted pyridines from isoxazolinones (Peters, R., et al. J. Org. Chem. 2015, 80, 6822).
The protocol involves a regioselective Pd(II)-catalyzed 1,4-addition of
isoxazolinones to enones, followed by a Pd(0)-catalyzed dihydropyridine
formation and oxidation. The formation of the dihydropyridine is
hypothesized via a vinylnitrene-Pd complex species formed by oxidative
addition of Pd(0) to the N–O bond of the 1,4-adduct followed by a
decarboxylation. This two-step sequence allows a rapid and
regioselective entry to substituted pyridines starting from readily
accessible isoxazolinones. Despite these advantages, the safety issue
needs to be addressed during application of this approach toward
large-scale production as the second step required a mixture of hydrogen
and air.
Subscribe to:
Posts (Atom)