A Stable Coordination Complex of Rh(IV) in an N,O-Donor Environment
http://pubs.acs.org/doi/abs/10.1021/jacs.5b12148
Shashi B. Sinha, Dimitar Y. Shopov, Liam S. Sharninghausen, David J. Vinyard, Brandon Q. Mercado, Gary W. Brudvig*, and Robert H. Crabtree*
Department of Chemistry, Yale University, 225 Prospect Street, New Haven, Connecticut 06520, United States
J. Am. Chem. Soc., Article ASAP
DOI: 10.1021/jacs.5b12148
Publication Date (Web): December 7, 2015
Copyright © 2015 American Chemical Society
Abstract: We describe facial and meridional isomers of [RhIII(pyalk)3], as well as meridional [RhIV(pyalk)3]+ {pyalk =2-(2-pyridyl)-2-propanoate}, the first coordination complex in an N,O-donor environment to show a clean, reversible RhIII/IV redox couple and to have a stable Rh(IV) form, which we characterize by EPR and UV–visible spectroscopy as well as X-ray crystallography. The unprecedented stability of the Rh(IV) species is ascribed to the exceptional donor strength of the ligands, their oxidation resistance, and the meridional coordination geometry.
Tuesday, December 15, 2015
Friday, December 11, 2015
Polyannulated Bis(N-heterocyclic carbene)palladium Pincer Complexes for Electrocatalytic CO2 Reduction
Polyannulated Bis(N-heterocyclic carbene)palladium Pincer
Complexes for Electrocatalytic CO2 Reduction
Jeffrey A. Therrien, Michael O. Wolf,* and Brian O. Patrick
Department of Chemistry, University of British Columbia, Vancouver, British Columbia V6T 1Z1, Canada
Inorg. Chem. DOI: 10.1021/acs.inorgchem.5b01698
Abstract:
Phenanthro- and pyreno-annulated N-heterocyclic carbenes (NHCs) have been incorporated into lutidine-linked bisNHC Pd pincer complexes to investigate the effect of these polyannulated NHCs on the ability of the complexes to electrochemically reduce CO2 to CO in the presence of 2,2,2-trifluoroacetic acid and 2,2,2-trifluoroethanol as proton sources. These complexes are screened for their ability to reduce CO2 and modeled using density functional theory calculations, where the annulated phenanthrene and pyrene moieties are shown to be additional sites for redox activity in the pincer ligand, enabling increased electron donation. Electrochemical and computational studies are used to gain an understanding of the chemical significance of redox events for complexes of this type, highlighting the importance of anion binding and dissociation.
TOC:
Jeffrey A. Therrien, Michael O. Wolf,* and Brian O. Patrick
Department of Chemistry, University of British Columbia, Vancouver, British Columbia V6T 1Z1, Canada
Inorg. Chem. DOI: 10.1021/acs.inorgchem.5b01698
Abstract:
Phenanthro- and pyreno-annulated N-heterocyclic carbenes (NHCs) have been incorporated into lutidine-linked bisNHC Pd pincer complexes to investigate the effect of these polyannulated NHCs on the ability of the complexes to electrochemically reduce CO2 to CO in the presence of 2,2,2-trifluoroacetic acid and 2,2,2-trifluoroethanol as proton sources. These complexes are screened for their ability to reduce CO2 and modeled using density functional theory calculations, where the annulated phenanthrene and pyrene moieties are shown to be additional sites for redox activity in the pincer ligand, enabling increased electron donation. Electrochemical and computational studies are used to gain an understanding of the chemical significance of redox events for complexes of this type, highlighting the importance of anion binding and dissociation.
TOC:
Standard Reduction Potentials for Oxygen and Carbon Dioxide Couples in Acetonitrile and N,N‑Dimethylformamide
Standard Reduction Potentials for Oxygen and Carbon Dioxide
Couples in Acetonitrile and N,N‑Dimethylformamide
Michael L. Pegis,† John A. S. Roberts,‡,∥ Derek J. Wasylenko,§,⊥ Elizabeth A. Mader,† Aaron M. Appel,*,‡ and James M. Mayer*,†
† Department of Chemistry, Yale University, PO Box 208107, New Haven, Connecticut 06520-8107, United States ‡ Center for Molecular Electrocatalysis, Pacific Northwest National Laboratory, P.O. Box 999 (K2-57), Richland, Washington 99352, United States § Department of Chemistry, University of Washington, Campus Box 351700, Seattle, Washington 98195-1700, United States
Inorg. Chem. DOI: 10.1021/acs.inorgchem.5b02136
Abstract:
A variety of next-generation energy processes utilize the electrochemical interconversions of dioxygen and water as the oxygen reduction reaction (ORR) and the oxygen evolution reaction (OER). Reported here are the first estimates of the standard reduction potential of the O2 + 4e − + 4H+ ⇋ 2H2O couple in organic solvents. The values are +1.21 V in acetonitrile (MeCN) and +0.60 V in N,N-dimethylformamide (DMF), each versus the ferrocenium/ferrocene couple (Fc+/0) in the respective solvent (as are all of the potentials reported here). The potentials have been determined using a thermochemical cycle that combines the free energy for transferring water from aqueous solution to organic solvent, −0.43 kcal mol−1 for MeCN and −1.47 kcal mol−1 for DMF, and the potential of the H+ /H2 couple, − 0.028 V in MeCN and −0.662 V in DMF. The H+ /H2 couple in DMF has been directly measured electrochemically using the previously reported procedure for the MeCN value. The thermochemical approach used for the O2/H2O couple has been extended to the CO2/CO and CO2/CH4 couples to give values of −0.12 and +0.15 V in MeCN and −0.73 and −0.48 V in DMF, respectively. Extensions to other reduction potentials are discussed. Additionally, the free energy for transfer of protons from water to organic solvent is estimated as +14 kcal mol−1 for acetonitrile and +0.6 kcal mol−1 for DMF.
TOC:
Michael L. Pegis,† John A. S. Roberts,‡,∥ Derek J. Wasylenko,§,⊥ Elizabeth A. Mader,† Aaron M. Appel,*,‡ and James M. Mayer*,†
† Department of Chemistry, Yale University, PO Box 208107, New Haven, Connecticut 06520-8107, United States ‡ Center for Molecular Electrocatalysis, Pacific Northwest National Laboratory, P.O. Box 999 (K2-57), Richland, Washington 99352, United States § Department of Chemistry, University of Washington, Campus Box 351700, Seattle, Washington 98195-1700, United States
Inorg. Chem. DOI: 10.1021/acs.inorgchem.5b02136
Abstract:
A variety of next-generation energy processes utilize the electrochemical interconversions of dioxygen and water as the oxygen reduction reaction (ORR) and the oxygen evolution reaction (OER). Reported here are the first estimates of the standard reduction potential of the O2 + 4e − + 4H+ ⇋ 2H2O couple in organic solvents. The values are +1.21 V in acetonitrile (MeCN) and +0.60 V in N,N-dimethylformamide (DMF), each versus the ferrocenium/ferrocene couple (Fc+/0) in the respective solvent (as are all of the potentials reported here). The potentials have been determined using a thermochemical cycle that combines the free energy for transferring water from aqueous solution to organic solvent, −0.43 kcal mol−1 for MeCN and −1.47 kcal mol−1 for DMF, and the potential of the H+ /H2 couple, − 0.028 V in MeCN and −0.662 V in DMF. The H+ /H2 couple in DMF has been directly measured electrochemically using the previously reported procedure for the MeCN value. The thermochemical approach used for the O2/H2O couple has been extended to the CO2/CO and CO2/CH4 couples to give values of −0.12 and +0.15 V in MeCN and −0.73 and −0.48 V in DMF, respectively. Extensions to other reduction potentials are discussed. Additionally, the free energy for transfer of protons from water to organic solvent is estimated as +14 kcal mol−1 for acetonitrile and +0.6 kcal mol−1 for DMF.
TOC:
Anhydrous Tetramethylammonium Fluoride for Room-Temperature SNAr Fluorination
Anhydrous Tetramethylammonium Fluoride for Room-Temperature
SNAr Fluorination
Sydonie D. Schimler,† Sarah J. Ryan,† Douglas C. Bland,‡ John E. Anderson,‡ and Melanie S. Sanford*,†
† Department of Chemistry, University of Michigan, 930 North University Avenue, Ann Arbor, Michigan 48109, United States ‡ Process Science, Dow Chemical Company, 1710 Building, Midland, Michigan 48674, United States
J. Org. Chem. DOI: 10.1021/acs.joc.5b02075
http://pubs.acs.org/doi/pdf/10.1021/acs.joc.5b02075
Abstract:
This paper describes the room-temperature SNAr fluorination of aryl halides and nitroarenes using anhydrous tetramethylammonium fluoride (NMe4F). This reagent effectively converts aryl-X (X = Cl, Br, I, NO2, OTf) to aryl-F under mild conditions (often room temperature). Substrates for this reaction include electron-deficient heteroaromatics (22 examples) and arenes (5 examples). The relative rates of the reactions vary with X as well as with the structure of the substrate. However, in general, substrates bearing X = NO2 or Br react fastest. In all cases examined, the yields of these reactions are comparable to or better than those obtained with CsF at elevated temperatures (i.e., more traditional halex fluorination conditions). The reactions also afford comparable yields on scales ranging from 100 mg to 10 g. A cost analysis is presented, which shows that fluorination with NMe4F is generally more costeffective than fluorination with CsF.
TOC:
Sydonie D. Schimler,† Sarah J. Ryan,† Douglas C. Bland,‡ John E. Anderson,‡ and Melanie S. Sanford*,†
† Department of Chemistry, University of Michigan, 930 North University Avenue, Ann Arbor, Michigan 48109, United States ‡ Process Science, Dow Chemical Company, 1710 Building, Midland, Michigan 48674, United States
J. Org. Chem. DOI: 10.1021/acs.joc.5b02075
http://pubs.acs.org/doi/pdf/10.1021/acs.joc.5b02075
Abstract:
This paper describes the room-temperature SNAr fluorination of aryl halides and nitroarenes using anhydrous tetramethylammonium fluoride (NMe4F). This reagent effectively converts aryl-X (X = Cl, Br, I, NO2, OTf) to aryl-F under mild conditions (often room temperature). Substrates for this reaction include electron-deficient heteroaromatics (22 examples) and arenes (5 examples). The relative rates of the reactions vary with X as well as with the structure of the substrate. However, in general, substrates bearing X = NO2 or Br react fastest. In all cases examined, the yields of these reactions are comparable to or better than those obtained with CsF at elevated temperatures (i.e., more traditional halex fluorination conditions). The reactions also afford comparable yields on scales ranging from 100 mg to 10 g. A cost analysis is presented, which shows that fluorination with NMe4F is generally more costeffective than fluorination with CsF.
TOC:
Wednesday, December 9, 2015
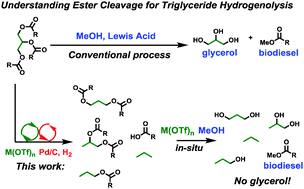
Mono- and tri-ester hydrogenolysis using tandem catalysis. Scope and mechanism
Mono- and tri-ester hydrogenolysis using tandem catalysis. Scope and mechanism
Tracy L. Lohr,a Zhi Li,a Rajeev S. Assary,b Larry A. Curtissbc and Tobin J. Marks*a
Energy Environ. Sci., 2016, Advance Article
DOI: 10.1039/C5EE03256C
Received 23 Oct 2015, Accepted 26 Nov 2015
First published online 26 Nov 2015
Abstract: The scope and mechanism of thermodynamically leveraged ester RC(O)O–R′ bond hydrogenolysis by tandem metal triflate + supported Pd catalysts are investigated both experimentally and theoretically by DFT and energy span analysis. This catalytic system has a broad scope, with relative cleavage rates scaling as, tertiary > secondary > primary ester at 1 bar H2, yielding alkanes and carboxylic acids with high conversion and selectivity. Benzylic and allylic esters display the highest activity. The rate law is ν = k[M(OTf)n]1[ester]0[H2]0 with an H/D kinetic isotope effect = 6.5 ± 0.5, implying turnover-limiting C–H scission following C–O cleavage, in agreement with theory. Intermediate alkene products are then rapidly hydrogenated. Applying this approach with the very active Hf(OTf)4 catalyst to bio-derived triglycerides affords near-quantitative yields of C3 hydrocarbons rather than glycerol. From model substrates, it is found that RC(O)O–R′ cleavage rates are very sensitive to steric congestion and metal triflate identity. For triglycerides, primary/external glyceryl CH2–O cleavage predominates over secondary/internal CH–O cleavage, with the latter favored by less acidic or smaller ionic radius metal triflates, raising the diester selectivity to as high as 48% with Ce(OTf)3.
Tuesday, December 8, 2015
Fighting Fenton Chemistry: A Highly Active Iron(III) Tetracarbene Complex in Epoxidation Catalysis
Jens W. Kück, Markus R. Anneser, Benjamin Hofmann, Dr. Alexander Pöthig, Dr. Mirza Cokoja, Prof. Dr. Fritz E. Kühn
http://onlinelibrary.wiley.com/doi/10.1002/cssc.201500930/full
Abstract
Organometallic Fe complexes with exceptionally high activities in homogeneous epoxidation catalysis are reported. The compounds display FeII and FeIII oxidation states and bear a tetracarbene ligand. The more active catalyst exhibits activities up to 183 000 turnovers per hour at room temperature and turnover numbers of up to 4300 at −30 °C. For the FeIII complex, a decreased Fenton-type reactivity is observed compared with FeII catalysts reported previously as indicated by a substantially lower H2O2 decomposition and higher (initial) turnover frequencies. The dependence of the catalyst performance on the catalyst loading, substrate, water addition, and the oxidant is investigated. Under all applied conditions, the advantageous nature of the use of the FeIII complex is evident.http://onlinelibrary.wiley.com/doi/10.1002/cssc.201500930/full
A manganese catalyst for highly reactive yet chemoselective intramolecular C(sp3)–H amination
Shauna M. Paradine, Jennifer R. Griffin, Jinpeng Zhao, Aaron L. Petronico, Shannon M. Miller, & M. Christina White
Nature Chemistry 7, 987–994 doi:10.1038/nchem.2366 Received Accepted Published online
A steric tethering approach enables palladium-catalysed C–H activation of primary amino alcohols
Jonas Calleja, Daniel Pla, Timothy W. Gorman, Victoriano Domingo, Benjamin Haffemayer, & Matthew J. Gaunt
Nature Chemistry 7, 1009–1016 doi:10.1038/nchem.2367 Received Accepted Published online
Abstract
Aliphatic primary amines are a class of chemical feedstock essential to the synthesis of higher-order nitrogen-containing molecules, commonly found in biologically active compounds and pharmaceutical agents. New methods for the construction of complex amines remain a continuous challenge to synthetic chemists. Here, we outline a general palladium-catalysed strategy for the functionalization of aliphatic C–H bonds within amino alcohols, an important class of small molecule. Central to this strategy is the temporary conversion of catalytically incompatible primary amino alcohols into hindered secondary amines that are capable of undergoing a sterically promoted palladium-catalysed C–H activation. Furthermore, a hydrogen bond between amine and catalyst intensifies interactions around the palladium and orients the aliphatic amine substituents in an ideal geometry for C–H activation. This catalytic method directly transforms simple, easily accessible amines into highly substituted, functionally concentrated and structurally diverse products, and can streamline the synthesis of biologically important amine-containing molecules.http://www.nature.com/nchem/journal/v7/n12/full/nchem.2367.html
Wednesday, December 2, 2015
Dual Role of Pyrrolidine and Cooperative Pyrrolidine/Pyrrolidinium Effect in Nitrone Formation
http://pubs.acs.org/doi/abs/10.1021/acscatal.5b01726
Sara Morales, Fernando G. Guijarro, Inés Alonso, José Luis García Ruano*, and M. Belén Cid*
Department of Organic Chemistry, Universidad Autónoma de Madrid, Cantoblanco, 28049 Madrid, Spain
ACS Catal., 2016, 6, pp 84–91
DOI: 10.1021/acscatal.5b01726
Publication Date (Web): November 12, 2015
Sara Morales, Fernando G. Guijarro, Inés Alonso, José Luis García Ruano*, and M. Belén Cid*
Department of Organic Chemistry, Universidad Autónoma de Madrid, Cantoblanco, 28049 Madrid, Spain
ACS Catal., 2016, 6, pp 84–91
DOI: 10.1021/acscatal.5b01726
Publication Date (Web): November 12, 2015
Abstract:
The formation of nitrones by direct condensation between equimolecular amounts of N-substituted hydroxylamine hydrochlorides and aromatic or aliphatic aldehydes is efficiently promoted by pyrrolidine in a matter of minutes under very mild conditions in almost quantitative yields after a simple filtration through a short pad of silica gel. According to theoretical, spectroscopic, and experimental studies, this success is due to the ability of pyrrolidine to liberate the hydrochloride of the hydroxylamine and catalyze the reaction via iminium activation ion. Moreover, a cooperative pyrrolidine/pyrrolidinium chloride effect facilitates several steps of the catalytic cycle through proton transfer without hampering the nucleophilicity of the hydroxylamine by protonation.
Keywords: nitrone preparation; iminiumactivation; cooperative; DFT calculations; pyrrolidine/pyrrolidinium
The formation of nitrones by direct condensation between equimolecular amounts of N-substituted hydroxylamine hydrochlorides and aromatic or aliphatic aldehydes is efficiently promoted by pyrrolidine in a matter of minutes under very mild conditions in almost quantitative yields after a simple filtration through a short pad of silica gel. According to theoretical, spectroscopic, and experimental studies, this success is due to the ability of pyrrolidine to liberate the hydrochloride of the hydroxylamine and catalyze the reaction via iminium activation ion. Moreover, a cooperative pyrrolidine/pyrrolidinium chloride effect facilitates several steps of the catalytic cycle through proton transfer without hampering the nucleophilicity of the hydroxylamine by protonation.
Keywords: nitrone preparation; iminiumactivation; cooperative; DFT calculations; pyrrolidine/pyrrolidinium
Synthesis, characterisation, and catalytic evaluation of hierarchical faujasite zeolites: milestones, challenges, and future directions†
Synthesis, characterisation, and catalytic
evaluation of hierarchical faujasite zeolites:
milestones, challenges, and future directions†
D. Verboekend,*a N. Nuttens,a R. Locus,a J. Van Aelst,a P. Verolme,b J. C. Groen,b J. Pe´rez-Ramı´rezc and B. F. Selsa
Chem. Soc. Rev. http://pubs.rsc.org/en/content/articlepdf/2016/cs/c5cs00520e?page=search
Abstract:
Faujasite (X, Y, and USY) zeolites represent one of the most widely-applied and abundant catalysts and sorbents in the chemical industry. In the last 5 years substantial progress was made in the synthesis, characterisation, and catalytic exploitation of hierarchically-structured variants of these zeolites. Hererin, we provide an overview of these contributions, highlighting the main advancements regarding the evaluation of the nature and functionality of introduced secondary porosity. The novelty, efficiency, versatility, and sustainability of the reported bottom-up and (predominately) top-down strategies are discussed. The crucial role of the relative stability of faujasites in aqueous media is highlighted. The interplay between the physico-chemical properties of the hierarchical zeolites and their use in petrochemical and biomass-related catalytic processes is assessed.
D. Verboekend,*a N. Nuttens,a R. Locus,a J. Van Aelst,a P. Verolme,b J. C. Groen,b J. Pe´rez-Ramı´rezc and B. F. Selsa
Chem. Soc. Rev. http://pubs.rsc.org/en/content/articlepdf/2016/cs/c5cs00520e?page=search
Abstract:
Faujasite (X, Y, and USY) zeolites represent one of the most widely-applied and abundant catalysts and sorbents in the chemical industry. In the last 5 years substantial progress was made in the synthesis, characterisation, and catalytic exploitation of hierarchically-structured variants of these zeolites. Hererin, we provide an overview of these contributions, highlighting the main advancements regarding the evaluation of the nature and functionality of introduced secondary porosity. The novelty, efficiency, versatility, and sustainability of the reported bottom-up and (predominately) top-down strategies are discussed. The crucial role of the relative stability of faujasites in aqueous media is highlighted. The interplay between the physico-chemical properties of the hierarchical zeolites and their use in petrochemical and biomass-related catalytic processes is assessed.
Heterogeneous chemistry and reaction dynamics of the atmospheric oxidants, O3, NO3, and OH, on organic surfaces
Heterogeneous chemistry and reaction dynamics
of the atmospheric oxidants, O3, NO3, and OH,
on organic surfaces
Robert C. Chapleski Jr., Yafen Zhang, Diego Troya and John R. Morris*
Chem. Soc. Rev. http://pubs.rsc.org/en/content/articlepdf/2016/cs/c5cs00375j?page=search
Abstract:
Heterogeneous chemistry of the most important atmospheric oxidants, O3, NO3, and OH, plays a central role in regulating atmospheric gas concentrations, processing aerosols, and aging materials. Recent experimental and computational studies have begun to reveal the detailed reaction mechanisms and kinetics for gas-phase O3, NO3, and OH when they impinge on organic surfaces. Through new research approaches that merge the fields of traditional surface science with atmospheric chemistry, researchers are developing an understanding for how surface structure and functionality affect interfacial chemistry with this class of highly oxidizing pollutants. Together with future research initiatives, these studies will provide a more complete description of atmospheric chemistry and help others more accurately predict the properties of aerosols, the environmental impact of interfacial oxidation, and the concentrations of tropospheric gases.
Robert C. Chapleski Jr., Yafen Zhang, Diego Troya and John R. Morris*
Chem. Soc. Rev. http://pubs.rsc.org/en/content/articlepdf/2016/cs/c5cs00375j?page=search
Abstract:
Heterogeneous chemistry of the most important atmospheric oxidants, O3, NO3, and OH, plays a central role in regulating atmospheric gas concentrations, processing aerosols, and aging materials. Recent experimental and computational studies have begun to reveal the detailed reaction mechanisms and kinetics for gas-phase O3, NO3, and OH when they impinge on organic surfaces. Through new research approaches that merge the fields of traditional surface science with atmospheric chemistry, researchers are developing an understanding for how surface structure and functionality affect interfacial chemistry with this class of highly oxidizing pollutants. Together with future research initiatives, these studies will provide a more complete description of atmospheric chemistry and help others more accurately predict the properties of aerosols, the environmental impact of interfacial oxidation, and the concentrations of tropospheric gases.
Subscribe to:
Posts (Atom)