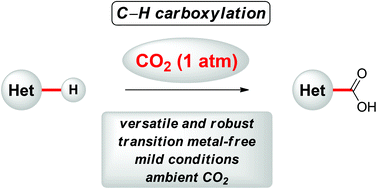
C–H carboxylation of heteroarenes with ambient CO2
Green Chem., 2016, Advance Article
The C–H carboxylation of heteroarenes was achieved under transition metal-free reaction conditions with naturally abundant CO2 as the C1 source at relatively low temperature. The C–H carboxylation was mediated by KOt-Bu at atmospheric pressure of CO2, and thereby provided atom- and step-economical access to various heteroaromatic carboxylic acid derivatives.