Mono- and tri-ester hydrogenolysis using tandem catalysis. Scope and mechanism
Tracy L. Lohr,a Zhi Li,a Rajeev S. Assary,b Larry A. Curtissbc and Tobin J. Marks*a
Energy Environ. Sci., 2016, Advance Article
DOI: 10.1039/C5EE03256C
Received 23 Oct 2015, Accepted 26 Nov 2015
First published online 26 Nov 2015
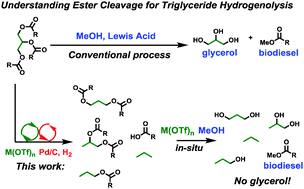
Abstract: The scope and mechanism of thermodynamically leveraged ester RC(O)O–R′ bond hydrogenolysis by tandem metal triflate + supported Pd catalysts are investigated both experimentally and theoretically by DFT and energy span analysis. This catalytic system has a broad scope, with relative cleavage rates scaling as, tertiary > secondary > primary ester at 1 bar H2, yielding alkanes and carboxylic acids with high conversion and selectivity. Benzylic and allylic esters display the highest activity. The rate law is ν = k[M(OTf)n]1[ester]0[H2]0 with an H/D kinetic isotope effect = 6.5 ± 0.5, implying turnover-limiting C–H scission following C–O cleavage, in agreement with theory. Intermediate alkene products are then rapidly hydrogenated. Applying this approach with the very active Hf(OTf)4 catalyst to bio-derived triglycerides affords near-quantitative yields of C3 hydrocarbons rather than glycerol. From model substrates, it is found that RC(O)O–R′ cleavage rates are very sensitive to steric congestion and metal triflate identity. For triglycerides, primary/external glyceryl CH2–O cleavage predominates over secondary/internal CH–O cleavage, with the latter favored by less acidic or smaller ionic radius metal triflates, raising the diester selectivity to as high as 48% with Ce(OTf)3.
No comments:
Post a Comment
Note: Only a member of this blog may post a comment.